The ocean takes up a large amount of the human-induced carbon dioxide in the atmosphere. This uptake changes the acidity level of sea water and the concentration of carbon ions which are necessary for calcium carbonate formation.
Calcium carbonate is found in the skeletons, or protective shells, of crustaceans, corals, snails, single-celled organisms, and bivalves. Decreasing concentrations of carbonate ions could negatively affect marine living conditions with still unknown implications for the oceanic food chain. In an extreme case, the carbonate shells of organisms could simply dissolve like lime scales treated with acidic cleaning agents in the bathroom.
The visualization shows the MPI-ESM simulation of carbonate saturation for calcite based on the pessimistic RCP8.5 scenario. The oversaturated water represented in red allows many oceanic life forms to build calcium carbonate skeletons and shells. The deep Pacific is the only area which holds undersaturated water. The situation undergoes drastic changes throughout the simulation, by the end of which, near 2300, one can see an overall large decrease in carbonate saturation; large areas from the middle latitudes to the pole regions are dominated by undersaturated water.
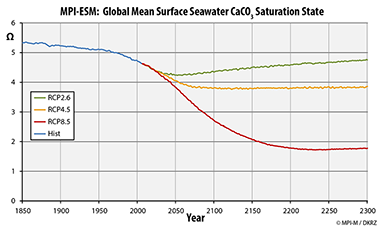
The graph on the right shows, for the time period from 1850 to 2300 and the scenarios RCP2.6, RCP4.5, and RCP8.5, the development of the global mean surface sea water saturation (Omega) for calcium carbonate (CaCO3). In the case of the pessimistic scenario RCP8.5, a strong decrease in the availability of calcite is simulated, while both other experiments result in less dramtic consequences. A moderate decrease in omega is simulated for he RPC4.5-simulation, followed by a stabilization at a value of about 3.8 until 2100. For RCP2.6, the calcite saturation state stabilizes earlier and then slowly recovers and returns to today's value until 2300.